Arterial blood gas
Introduction:
Interpretation of arterial blood gas (ABG) is crucial for physicians, nursing staff, and other health care professionals. ABG interpretation is specifically important for the management of critically ill patients.
ABG report:
ABG report provides information about Acid-base and oxygenation status.
Bicarbonate of ABG report is calculated on the basis of the Henderson-Hasselbalch (HH) equation.
pH = 6.1 + log HCO3/0.03 x PCO2
pH, PaCO2, and HCO3 give an overview of acid-base status and PaO2 gives an overview of oxygenation.
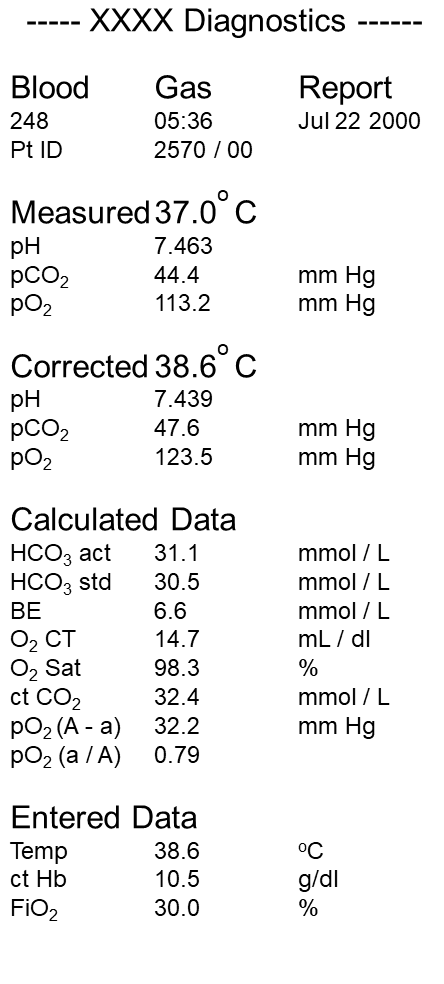
ABG report provides a few measured parameters and other calculated parameters.
Measured parameters are
- pH
- pCO2
- pO2.
Calculated parameters are
- HCO3
- BE
- O2 saturation.
Machine calculate and correct this parameter on the basis of entered values like
- Body temperature
- Hemoglobin (Newer machine calculated hemoglobin from the provided sample).
- Fraction of inspired oxygen (FiO2).
Hence it is important to provide data of the patient for accurate reporting.
Acid-Base in ABG
pH and PaCO2 used for evaluation of Acid-Base status of the body. Bicarbonate and base excess is derived from predefined calculations (As shown above).
Rules of ABG interpretation:
- pH increases in alkalemia and decreases in acidemia.
- PaCO2 is acidic. Hence when increases respiratory acidemia increases and vice versa.
- HCO3- is alkalic. Hence when increases metabolic alkalemia increases and vice versa.
- HCO3 and PaCO2 act as buffers by compensating for abnormalities of each other.
- Respiratory acidemia (Increase PaCO2) is compensated by metabolic alkalemia (increase HCO3).
- Respiratory alkalemia (decrease PaCO2) is compensated by metabolic acidemia (decrease HCO3).
- Metabolic alkalemia (increase HCO3) is compensated by respiratory acidemia (increase PaCO2).
- Metabolic acidemia (decrease HCO3) is compensated by respiratory alkalemia (decrease PaCO2).
- The individual normal value of any of the above doesn’t suggest normalcy.
↓ PaCO2 ⇒ ↑pH
↑ PaCO2 ⇒ ↓ pH
↑ HCO3 ⇒ ↑ pH
↓ HCO3 ⇒ ↓ pH
Normal range:
- pH – 7.35 to 7.45
- Partial pressure of CO2 (PaCO2) – 35 – 45 mmHg.
- Bicarbonate (HCO3) – 22 – 26 meq/L or mmol/L.
Steps - Evaluation of acid-base status of ABG
Step 1: pH
- pH < 7.35 suggest Acidemia.
- pH > 7.45 suggest Alkalemia.
- Normal pH does not rule out an ABG abnormality.
Step 2: Who is responsible for the change in pH?
Look for PaCO2 value: Is the value abnormal?
If carbon dioxide is primarily responsible for the change in pH, then the pH will change in the opposite direction of the change in PaCO2.
(High PaCO2 should lower pH and low PaCO2 will increase pH).
Look for bicarbonate: Is the value abnormal?
If bicarbonate is primarily responsible for the change in pH, then the pH will change in the direction of the change in bicarbonate.
(Low bicarbonate should lower pH and high bicarbonate will increase pH).
↑pH + ↓ PaCO2 ⇒ Respiratory alkalosis
↓ pH + ↑ PaCO2 ⇒ Respiratory acidosis
↑ pH + ↑ HCO3 ⇒ Metabolic alkalosis
↓ pH + ↓ HCO3 ⇒ Metabolic acidosis
At the end of this step, primary ABG abnormality is diagnosed.
e.g.:
Acidosis :
pH <7.35 with HCO3 < 22 = Metabolic acidosis.
OR
pH <7.35 with PaCO2 > 42 = Respiratory acidosis.
Alkalosis :
pH >7.45 with bicarbonate > 26 = Metabolic alkalosis.
OR
pH >7.45 with PaCO2 < 38 = Respiratory alkalosis.
Mixed abnormality:
Acidosis – pH <7.35:
with bicarbonate < 22 and PaCO2 > 42 = Metabolic acidosis with respiratory acidosis.
Alkalosis – pH >7.45:
with bicarbonate > 26 and PaCO2 < 38 = Metabolic alkalosis with respiratory alkalosis.
↑pH + ↓ PaCO2 + ↑ HCO3 ⇒ Respiratory alkalosis with metabolic alkalosis
↓ pH + ↑ PaCO2 + ↓ HCO3 ⇒ Respiratory acidosis with metabolic acidosis
Step 3: Look for compensation
As described above HCO3 (metabolic) or PaCO2 (respiratory) act as a buffer. They compensate for each other. But there is a limit of compensation that this buffer can provide. Crossing this limit leads to mixed abnormality.
Metabolic acidosis (decrease in HCO3)
The respiratory system compensates for metabolic acidosis by decreasing PaCO2.
Following formulae is used for calculating the expected PaCO2 level for the level of metabolic acidosis.
Expected PaCO2 = (1.5 x HCO3) + 8 ± 2.
After calculating the expected PaCO2, compare the value with the reported or measured PaCO2.
- Reported PaCO2 more than expected PaCO2 => Additional Respiratory Acidosis.
- Measured or reported PaCO2 equals expected PaCO2 => Compensated Metabolic Acidosis.
- Reported PaCO2 is less than expected PaCO2 => Additional Respiratory Alkalosis.
Metabolic alkalosis (increase in HCO3)
Following formulae is used for calculating the expected PaCO2 level for the level of metabolic alkalosis.
Expected PaCO2 = 0.7 x (HCO3 – 24) +40 + 2
After calculating the expected PaCO2, compare the value with the reported PaCO2.
- Reported PaCO2 is more than expected PaCO2 ⇒ Additional Respiratory Acidosis.
- Measured or reported PaCO2 equals expected PaCO2 ⇒ Compensated Metabolic Alkalosis.
- Reported PaCO2 is less than expected PaCO2 ⇒ Additional Respiratory Alkalosis.
Respiratory acidosis (increase in PaCO2)
Compensation in respiratory abnormality can be judged by correlating change in HCO3– with change in PaCO2 above/below 40 mmHg.
With every 10mmHg increase in PaCO2 above 40, increase in HCO3 by
less than 1 ⇒ Additional metabolic acidosis
- equal to 1 ⇒ Acute respiratory acidosis.
- in range of >1 to <4 ⇒ Partially compensated respiratory acidosis.
- in range of 4 to 5 ⇒ Chronic respiratory acidosis.
- more than 5 ⇒ Additional metabolic alkalosis.
Respiratory alkalosis (decrease in PaCO2)
With every 10mmhg decrease in PaCO2 below 40, decrease in HCO3 by:
- less than 2 ⇒ Additional metabolic alkalosis.
equal to 2 ⇒ Acute respiratory alkalosis. - in range of >2 to <4 ⇒ Partially compensated respiratory alkalosis.
- in range of 4 to 5 ⇒ Chronic respiratory alkalosis.
- more than 5 ⇒ Additional metabolic acidosis.
- less than 2 ⇒ Additional metabolic alkalosis.
Step 4: Clinical correlation
No arterial blood gas assessment is complete without clinical correlation.
Alveolar-Arterial gradient (AaDO2)
The alveolar-arterial gradient is covered in the next article. To read please click here.
